Chitosan for vaccine development against SARS-CoV-2
The global spread of SARS-CoV-2 can only be contained through vaccine development. Vector vaccines (DNA and viral) can be produced rapidly and inexpensively with current synthesis technologies. Challenges with DNA vaccines include degradation by DNases, inefficient uptake by antigen-presenting cells, and low immunogenicity. The Quil-A-loaded chitosan particulate adjuvant system (QAC) enables transport of plasmid DNA directly to target cells and delayed release over time. Here, we present two recent studies about chitosan-based delivery systems for vaccines.
Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization.
Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.;Wu, C.-W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M., Vaccines 2021, 9, 132. https://doi.org/10.3390/vaccines9020132
The authors had previously studied Quil-A-loaded chitosan (QAC) nanoparticles in combination with plasmid vaccines against avian coronavirus and were able to demonstrate a robust immune response. In the presented study, the immune response to a mucosal homologous plasmid and a heterologous immunization strategy using a plasmid vaccine and the modified vaccinia Ankara (MVA), which expresses SARS-CoV-2 spike (S) and nucleocapsid (N) antigens, was investigated in mice. Modified vaccinia ankara virus (MVA) is an attenuated cowpox virus used as a viral vector. Efficient large-scale production is already possible and could be used to combat the COVID-19 pandemic. The authors hypothesize that a combination of 1st vaccination with the QAC-encapsulated plasmid DNA (primer) and 2nd vaccination with an MVA-boost elicits a broader immune response (heterologous strategy).
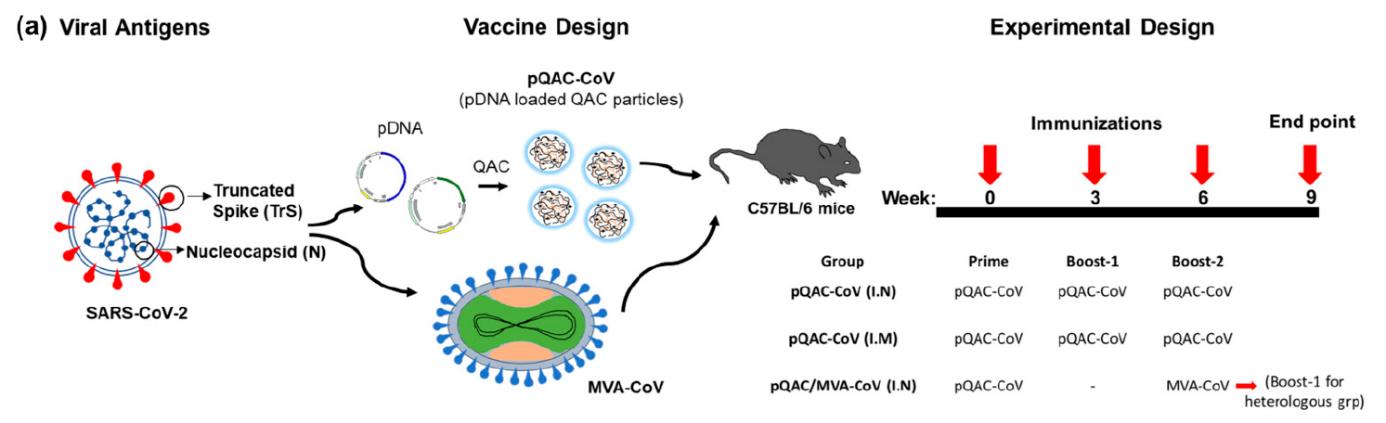
Figure 1: Viral antigens, vaccine design and experimental design. Source: Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization. Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.;Wu, C.-W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M., Vaccines 2021, 9, 132. https://doi.org/10.3390/vaccines9020132
Results
- Neutralizing antibodies in serum and bronchoalveolar lavage of mice only with the heterologous intranasal immunization strategy.
- Induction of type-1 and type-17 T-cell responses.
- Induction of type 17 T-cell responses (expression of type 1 cytokines) in lung and spleen.
- The plasmid homologous vaccination strategy induced the induction of local mono- and polyfunctional T cells secreting IFN- γ
Conclusion: The study results highlight the potential of QAC nano-vaccines to elicit a significant immune response against novel coronavirus. The heterologous vaccination strategy appears to be more suitable for generating immunity. The homologous vaccination strategy probably could not prevent SARS-CoV-2 infection, but probably could prevent a severe course. Further studies are needed to conclusively assess the efficacy and potential of the plasmid DNA-loaded Quil-A chitosan nanoparticles.
Source: https://pubmed.ncbi.nlm.nih.gov/33562141/
In Vitro Characterization of Inhalable Cationic Hybrid Nanoparticles as Potential Vaccine Carriers.
Alfagih, I.M.; Kaneko, K.; Kunda, N.K.; Alanazi, F.; Dennison, S.R.; Tawfeek, H.M.; Saleem, I.Y. Pharmaceuticals 2021, 14, 164. https://doi.org/10.3390/ph14020164
Delivery of vaccines via the lungs would mimic the natural route of infection of pulmonary pathogens and could thereby elicit a local immune response. The lung provides antigen-presenting cells, such as dendritic cells, and a large surface area for drug absorption. For pulmonary vaccine delivery, suitable formulations that offer appropriate deliverability, biocompatibility, and immunogenicity are needed. Nanoparticles consisting of poly(glycerol adipate-co-ω-pentadecalactone) (PGAco- PDL) could serve as transport vehicles.
The researcher’s prepared bovine serum albumin (BSA)-filled PGA-co-PDL nanoparticles (NPs) by emulsion solvent evaporation. In addition, chitosan hydrochloride (HCL) was introduced into the outer phase for surface absorption on the nanoparticles. The BSA-filled cationic chitosan HCl nanoparticles were incorporated into nanocomposite microcarriers (NCMPs) of L-leucine and spray-dried. The chitosan HCl NPs/NCMPs were evaluated in various assays for in vitro aerosolization, release, cell viability, uptake by cells, and protein structural stability. The authors used chitosan HCl with a deacetylation degree of 80-95% and a molecular weight in the range of 200 to 400 kDa from Heppe Medical Chitosan.
Results
- Successful preparation of hybrid cationic chitosan HCl NPs with particle sizes of 480.2±32.2 nm, charge of +14.2±0.72 mV and loading of BSA of 7.28±1.3 µg/mg.
- Formation of fine particle fractions
- Stable BSA protein structure after release from the chitosan HCl NPs/NCMPs.
- Almost complete release after 48h
- Cell viability of 70% in dendritic cells and A549 cells at a concentration of 2.5 mg/ml after 4-24h exposure (MTT assay)
- Uptake of chitosan HCl NPs by dendritic cells within one hour.
- Activation of dendritic cells by the chitosan HCl NPs (expression of CD40, CD86 and MHC-II cell surface markers)
Conclusion: The study results demonstrate the potential of the Chitosan HCl NPs/NCMPs platform for drug delivery into the lung and immune stimulatory applications such as vaccines. The multilayer surface adsorption of chitosan HCl enables the production of positively charged nanoparticles. Further studies are needed to test the drug release system in animal models.


