Chitin

Chitin belongs to the biopolymer group and its fibrous structure is similar to cellulose.
| Chemical name: | (1,4)-N-acetyl-D-glucos-2-amine |
| Empirical formula: | (C8H13NO5)n |
| CAS: | 1398-61-4 |
The monomers are identified as N-Acetyl-Amnioglucose. Chitin is a polysaccharide containing nitrogen in which monomers occur with the glycosidically linked components beta 1,4. It is the same coupling as glucose with cellulose, however in chitin the hydroxyl group of the monomer is replaced with an acetyl amine group. The resulting, stronger hydrogen bond between the bordering polymers makes chitin harder and more stabile than cellulose.

Structure Chitin / Chitosan: 50% deacetylizated
|
Chitin was first isolated and characterized in 1811 by the chemist and botanist Henry Braconnot. |
Occurrence in nature
- in the exoskeleton of animals such as shrimp, crabs, krill, squid and insects or in cell walls of fungi, yeast and other microorganisms.
- the amount of chitin in marine biomass alone is approx. 106-107 tons.
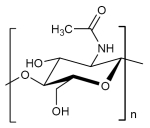
Structural formula Chitin
|
A distinction is made between alpha, beta and gamma chitin, which have different mechanical properties depending on the arrangement of the polymer chains.
|
Function of chitin in nature
- supporting function (structural substance)
- protection of soft parts (offers protection to inner organs)
- prevention of loss of fluids
Solubility
- insoluble in water, organic solvents, weak acids and lyes
- soluble in concentrated formic acid and methane sulfonic acid,
- strong acids split chitin into acetic acid and D-amino glucose (monomer of chitin), strong lyes split chitin into acetic acid and chitosan.
Biodegradability
- through the enzymes chitinases and lysozyme to chitobioses and through the chitobiases to monosaccharides
Applications
Chitin can be processed further into different derivatives. The two main derivatives are chitosan and amino glucose.
Chitin applications in cosmetics
- as carboxymethyl chitin (moisturizers, changes flow properties), antistatic effect due to cationic properties (hair products)
Chitin applications in medicine / pharmaceutics
- wound and burn treatment
- hemostatic for orthopedic treatment of broken bones
- viscoelastic solutions for ophthamology and orthopedic surgery
- abdominal adhesions treatment
- as antibacterial and antifungal agents and for treatment of mucous membranes
- in tumor therapies
- in micro surgery, neurosurgery
- for treatment of chronic wounds, ulcers and bleeding (chitin powder)
Supply of chitin
In our chitin and chitosan online store you can order chitin from different raw material sources.
Do you wish a personal offer? Please contact the


